Tag Archive for: Engineering
 https://www.college.ucla.edu/wp-content/uploads/2024/12/thumbnail-magic-363-241-wordpress-2.png
241
363
Alvaro Castillo
https://www.college.ucla.edu/wp-content/uploads/2019/07/Uxd_Blk_College-e1557344896161.png
Alvaro Castillo2024-12-12 03:39:362024-12-19 10:22:54What is magic? Defining the indefinable through a UCLA glass, darkly
https://www.college.ucla.edu/wp-content/uploads/2024/12/thumbnail-magic-363-241-wordpress-2.png
241
363
Alvaro Castillo
https://www.college.ucla.edu/wp-content/uploads/2019/07/Uxd_Blk_College-e1557344896161.png
Alvaro Castillo2024-12-12 03:39:362024-12-19 10:22:54What is magic? Defining the indefinable through a UCLA glass, darkly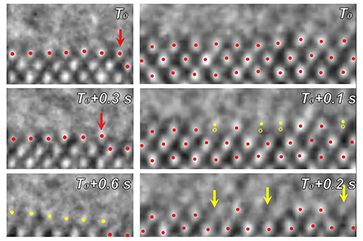 https://www.college.ucla.edu/wp-content/uploads/2024/07/atomiccrop_363-241.png
241
363
Alvaro Castillo
https://www.college.ucla.edu/wp-content/uploads/2019/07/Uxd_Blk_College-e1557344896161.png
Alvaro Castillo2024-06-20 11:20:522024-07-03 11:21:34Atomic view of a chemical catalyst during electrically charged reaction is a scientific first
https://www.college.ucla.edu/wp-content/uploads/2024/07/atomiccrop_363-241.png
241
363
Alvaro Castillo
https://www.college.ucla.edu/wp-content/uploads/2019/07/Uxd_Blk_College-e1557344896161.png
Alvaro Castillo2024-06-20 11:20:522024-07-03 11:21:34Atomic view of a chemical catalyst during electrically charged reaction is a scientific first https://www.college.ucla.edu/wp-content/uploads/2024/04/ChuckLorreAdrianaGalvanGneBlock-363-241.png
241
363
Alvaro Castillo
https://www.college.ucla.edu/wp-content/uploads/2019/07/Uxd_Blk_College-e1557344896161.png
Alvaro Castillo2024-03-26 08:35:582024-04-04 08:36:31Bloomberg Businessweek: UCLA receives a A $24.5 million pledge from The Chuck Lorre Family Foundation
https://www.college.ucla.edu/wp-content/uploads/2024/04/ChuckLorreAdrianaGalvanGneBlock-363-241.png
241
363
Alvaro Castillo
https://www.college.ucla.edu/wp-content/uploads/2019/07/Uxd_Blk_College-e1557344896161.png
Alvaro Castillo2024-03-26 08:35:582024-04-04 08:36:31Bloomberg Businessweek: UCLA receives a A $24.5 million pledge from The Chuck Lorre Family Foundation https://www.college.ucla.edu/wp-content/uploads/2024/03/Lorre-crop-1500-630.png
630
1500
Alvaro Castillo
https://www.college.ucla.edu/wp-content/uploads/2019/07/Uxd_Blk_College-e1557344896161.png
Alvaro Castillo2024-03-04 14:54:012024-03-05 17:49:27An even Bigger Bang: Chuck Lorre’s $24.5 million gift to empower low-income STEM students
https://www.college.ucla.edu/wp-content/uploads/2024/03/Lorre-crop-1500-630.png
630
1500
Alvaro Castillo
https://www.college.ucla.edu/wp-content/uploads/2019/07/Uxd_Blk_College-e1557344896161.png
Alvaro Castillo2024-03-04 14:54:012024-03-05 17:49:27An even Bigger Bang: Chuck Lorre’s $24.5 million gift to empower low-income STEM students https://www.college.ucla.edu/wp-content/uploads/2024/02/FulbrightImage-1500-630.png
630
1500
Alvaro Castillo
https://www.college.ucla.edu/wp-content/uploads/2019/07/Uxd_Blk_College-e1557344896161.png
Alvaro Castillo2024-02-13 12:10:542024-02-28 11:05:02Three UCLA College professors among UCLA’s Fulbright U.S. Scholars for 2023–24
https://www.college.ucla.edu/wp-content/uploads/2024/02/FulbrightImage-1500-630.png
630
1500
Alvaro Castillo
https://www.college.ucla.edu/wp-content/uploads/2019/07/Uxd_Blk_College-e1557344896161.png
Alvaro Castillo2024-02-13 12:10:542024-02-28 11:05:02Three UCLA College professors among UCLA’s Fulbright U.S. Scholars for 2023–24 https://www.college.ucla.edu/wp-content/uploads/2024/02/NAE-Electees-363-241.png
241
363
Alvaro Castillo
https://www.college.ucla.edu/wp-content/uploads/2019/07/Uxd_Blk_College-e1557344896161.png
Alvaro Castillo2024-02-06 11:40:272024-02-20 10:36:39Professor Rong Fu is one of three UCLA faculty elected to National Academy of Engineering
https://www.college.ucla.edu/wp-content/uploads/2024/02/NAE-Electees-363-241.png
241
363
Alvaro Castillo
https://www.college.ucla.edu/wp-content/uploads/2019/07/Uxd_Blk_College-e1557344896161.png
Alvaro Castillo2024-02-06 11:40:272024-02-20 10:36:39Professor Rong Fu is one of three UCLA faculty elected to National Academy of Engineering https://www.college.ucla.edu/wp-content/uploads/2024/01/Quantum-Machine-363-241.png
241
363
Alvaro Castillo
https://www.college.ucla.edu/wp-content/uploads/2019/07/Uxd_Blk_College-e1557344896161.png
Alvaro Castillo2024-01-03 14:42:512024-02-20 10:25:35The UCLA Research Park: Quantum science and engineering
https://www.college.ucla.edu/wp-content/uploads/2024/01/Quantum-Machine-363-241.png
241
363
Alvaro Castillo
https://www.college.ucla.edu/wp-content/uploads/2019/07/Uxd_Blk_College-e1557344896161.png
Alvaro Castillo2024-01-03 14:42:512024-02-20 10:25:35The UCLA Research Park: Quantum science and engineering https://www.college.ucla.edu/wp-content/uploads/2023/11/spacebruin-363-241.png
241
363
Alvaro Castillo
https://www.college.ucla.edu/wp-content/uploads/2019/07/Uxd_Blk_College-e1557344896161.png
Alvaro Castillo2023-11-07 15:18:372024-02-14 21:40:21UCLA: A Space Odyssey
https://www.college.ucla.edu/wp-content/uploads/2023/11/spacebruin-363-241.png
241
363
Alvaro Castillo
https://www.college.ucla.edu/wp-content/uploads/2019/07/Uxd_Blk_College-e1557344896161.png
Alvaro Castillo2023-11-07 15:18:372024-02-14 21:40:21UCLA: A Space Odyssey https://www.college.ucla.edu/wp-content/uploads/2023/09/Firefighter-363-241.jpg
241
363
Alvaro Castillo
https://www.college.ucla.edu/wp-content/uploads/2019/07/Uxd_Blk_College-e1557344896161.png
Alvaro Castillo2023-09-01 15:10:132023-09-01 15:10:13UCLA-led climate projects receive $7.5 million in state-funded grants from UC
https://www.college.ucla.edu/wp-content/uploads/2023/09/Firefighter-363-241.jpg
241
363
Alvaro Castillo
https://www.college.ucla.edu/wp-content/uploads/2019/07/Uxd_Blk_College-e1557344896161.png
Alvaro Castillo2023-09-01 15:10:132023-09-01 15:10:13UCLA-led climate projects receive $7.5 million in state-funded grants from UC
The nature of innovation
Marine scientist Kelsi Rutledge explores new possibilities for…

